Orthofix has got FDA clearance for its Fitbone™ Transport and Lengthening System.This medical device is designed to address large bone defects in the femur and tibia resulting from trauma, infection or malignant conditions, through a single surgical procedure.
The Fitbone intramedullary lengthening nail, acquired from Wittenstein SE in March 2020, is a fully implantable system for correcting leg length and deformity discrepancies. Implanted through a minimally invasive procedure, the system consists of the motorized intramedullary nail, a subcutaneously placed receiver and an external control set that enables the patient to manage the distraction phase at home. Once the treatment is complete, the nail and receiver are removed.
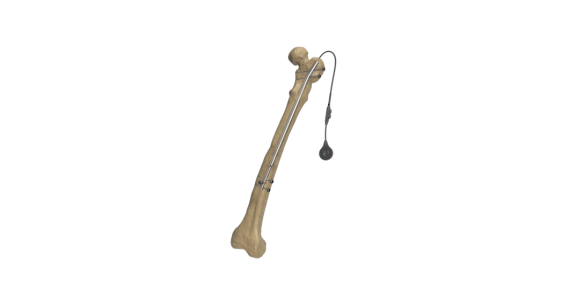
Limb alignment and lengthening technology
The Fitbone™ intramedullary lengthening nail, developed by Prof. Rainer Baumgart in Germany, has an electrical motor but no battery. Instead, the motorized drive is hermetically encapsulated in an induction coil and does not have contact with the exterior, so the patient’s skin can be fully closed once the surgery ends. The motor is powered by electricity transmitted by a second induction coil placed on the exterior surface of the body, adjacent to the internal coil. This electrical supply can be supplied either intermittently three to four times a day.
The creator of the Fitbone™ also proposed a new method of surgical planning, devised to achieve lengthening without creating deformity. With this approach, called reverse planning method, the planning starts with the intended final result of the projected lengthening, working backward to the preoperative status. The locking position and the nail diameter are taken into consideration to provide a final result in which all geometrical values are achieved, including the mechanical axes, equality of limb length and torsion, as well as correct physiological alignment of joints.
Regarding lower limb lengthening, the Fitbone™ system can be used in both the femur and tibia, either using the antegrade and retrograde approach in the femur and the antegrade approach in the tibia. Briefly, the SAA has a straight construction, with a sliding longitudinal hole in its middle, allowing for lengthening and bone transport, while the TAA is available in both a straight version and one with a Herzog bending for the tibia.
Important milestones
In Mar 2023,Orthofix announced Fitbone™ TAA intramedullary limb-lengthening system has passed 5,000 devices implanted to date with more than 20 years of clinical history demonstrating safety and effectiveness in limb lengthening and deformity correction in adults and children.
The Fitbone intramedullary system has more than 20 years of clinical history demonstrating safety and effectiveness in limb lengthening and deformity correction in adults and children
About Orthofix
The newly merged Orthofix-SeaSpine organization is a leading global spine and orthopedics company with a comprehensive portfolio of biologics, innovative spinal hardware, bone growth therapies, specialized orthopedic solutions and a leading surgical navigation system.Its products are distributed in approximately 68 countries worldwide.
Nota:The information on this site has been included in good faith for general informational purposes only.